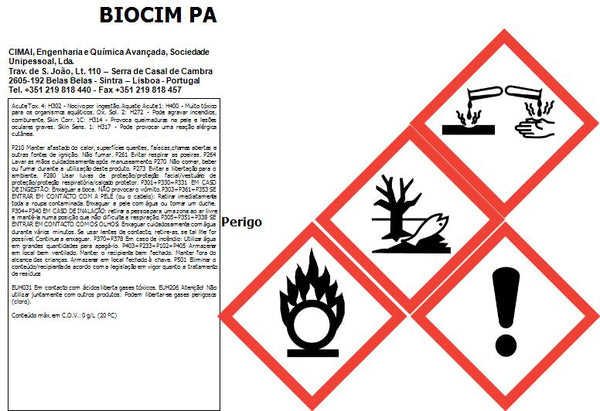
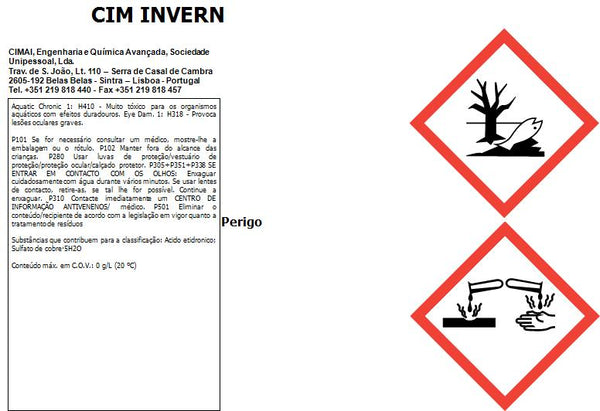
Not sterile. Class I medical device, non-sterile, in compliance with regulation (UE) 2017/745.
Waterproof.
Purpose intended: Protection of health professionals and patients against possible contamination, with a view to preventing disease or damage, in a hospital, industrial, laboratory or other environment, namely pool docks.
Siingle Size
For environmental and safety issues of our customers, CIMAI does not provide the technical data sheets and safety data sheets on paper, and all this documentation is available in PDF online on our website, and can even be downloaded.
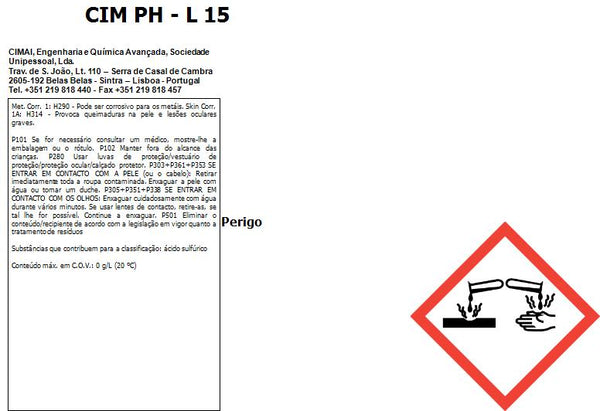
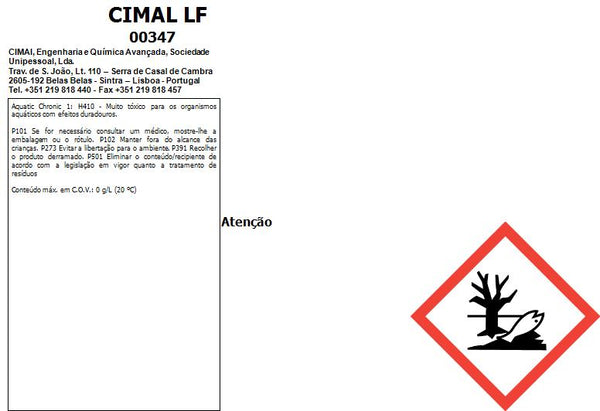
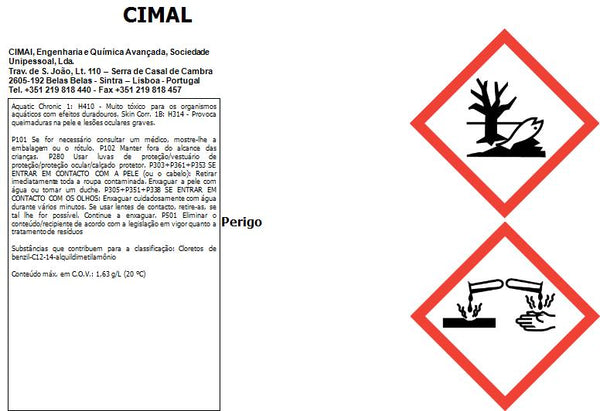
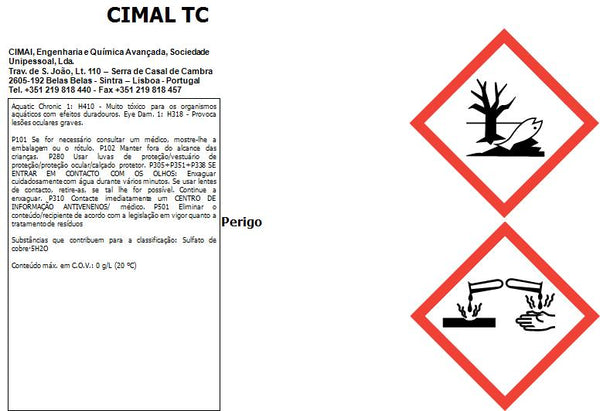
Please consult the documents for proper and safe use (Technical data and Safety data sheet).
Please consult the documents for proper and safe use (Technical data and Safety data sheet).
Delivery time - up to 48 hours for products without ADR (products that are not part of the European Treaty on dangerous goods) and up to 78 hours for products with ADR.
ADR products have a higher level of danger, which means that they have to be transported in special vehicles that must also be driven by properly trained drivers and with a specific license. These transports are rarer than the standard ones and deliveries are usually longer, as there are incompatible products between them, which cannot therefore be transported together.
Shipping costs depend on the weight and/or volume of your order. Please refer to the tables below.
ADR products have a higher level of danger, which means that they have to be transported in special vehicles that must also be driven by properly trained drivers and with a specific license. These transports are rarer than the standard ones and deliveries are usually longer, as there are incompatible products between them, which cannot therefore be transported together.
Shipping costs depend on the weight and/or volume of your order. Please refer to the tables below.
Related Products
CIMAI
PCO
PCO
PCO
PCO
PCO
PCO
PCO
PCO
PCO
PCO
PCO